Research
Revealing mitochondrial mechanisms underlying cardiac health and pathology.
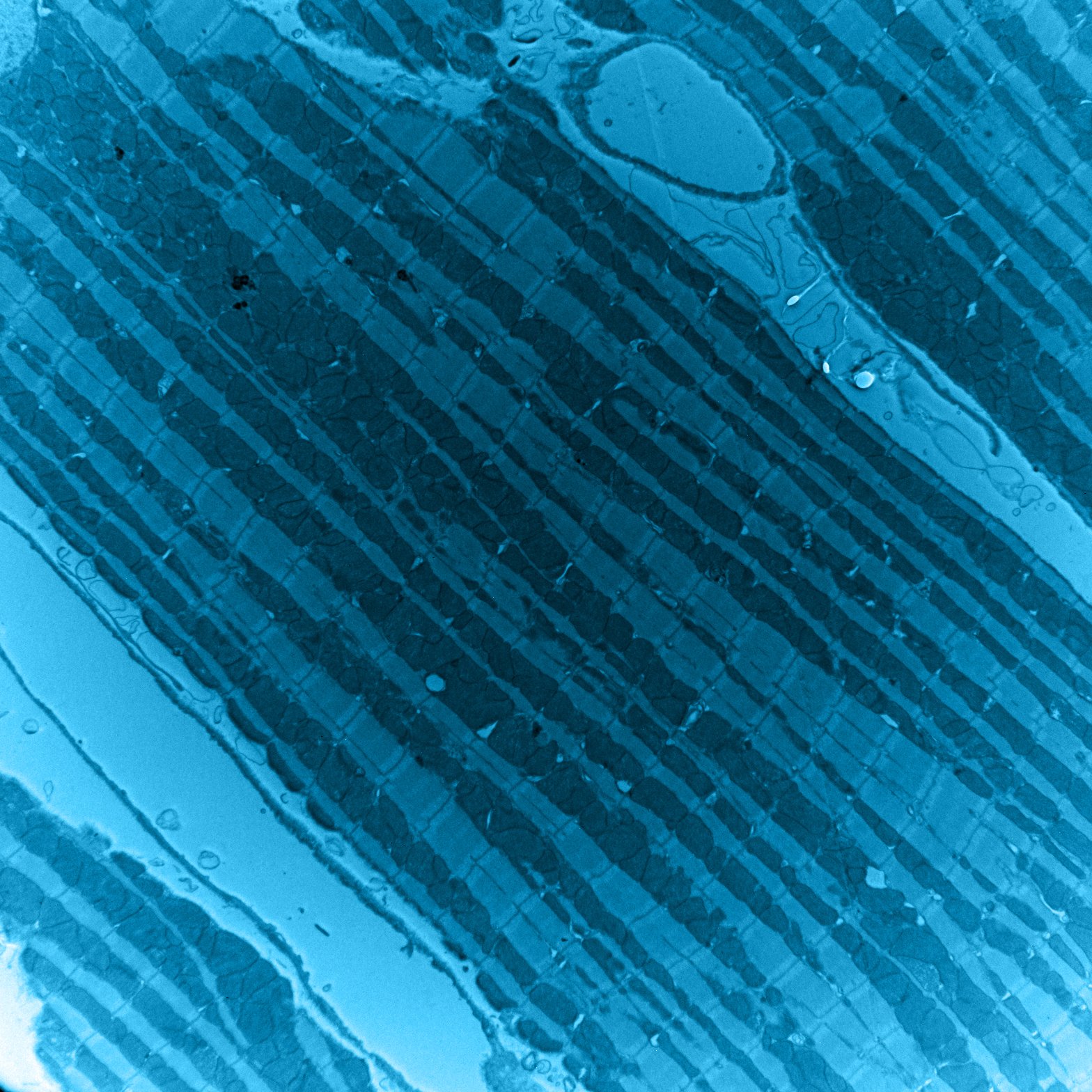
Research in the Kwong Lab is aimed toward uncovering basic mechanisms regulating mitochondrial bioenergetics, pathways underlying mitochondrial communication of energy dysfunction, and how these mechanisms and pathways regulate heart function and development. Leveraging genetically modified mouse models, cell biology, and biochemistry-based approaches, the goal of our research is to understand how mitochondria-mediated mechanisms contribute to cardiac diseases including primary mitochondrial disorders, mitochondrial cardiomyopathy, congenital heart disease, and heart failure.
Acylations: a novel pathway in the response to mitochondrial energy dysfunction.
Primary mitochondrial disorders arising from primary defects in the mitochondrial energy production machinery are the most common form of inborn errors of metabolism and often impact high-energy tissues like the heart. Approximately one-third of mitochondrial disease patients present with cardiac diseases such as hypertrophic cardiomyopathies. To date, there is no cure for mitochondrial disorders and no therapies designed specifically to address associated cardiomyopathy. Identifying molecular pathways activated in response to mitochondria energy defects may hold the key to the development of therapies for these incurable disorders.
This project leverages a mouse model with cardiomyocyte-specific and inducible deletion of the mitochondrial phosphate carrier (SLC25A3) as a novel approach to study the molecular consequences of mitochondrial energy dysfunction in the heart. Cardiac-specific SLC25A3-deleted mice have impaired ATP synthesis and develop severe hypertrophic cardiomyopathy similar to that in human patients with mitochondrial phosphate carrier deficiency (MPCD) caused by SLC25A3 mutations.
We discovered that SLC25A3 deletion-induced mitochondrial energy dysfunction results in a striking and distinct signature of acylome remodeling, with a progressive accumulation of post-translational acetylation and malonylation on mitochondrial proteins. Ongoing work in the lab is aimed at delineating the mechanisms underlying SLC25A3 deletion/mitochondrial energy dysfunction-induced mitochondrial acetylome and malonylome remodeling and the consequences of these modifications on cardiac function and disease.
The mitochondrial citrate carrier as a novel player in congenital heart disease.
Congenital heart disease (CHD) is the most common type of birth defect. CHD comprises structural and functional malformations of the heart present at birth that can require surgery or lifelong care. While a number of risk factors for CHD have been identified, much remains unknown about the underlying mechanisms and genetic pathways. 22q11.2 deletion syndrome (22q11DS) is a devastating multisystem disorder caused by microdeletions within chromosome 22q11.2 as well as the most common known genetic risk factor for CHD.
Through a recent collaboration, we identified the mitochondrial citrate carrier (Slc25a1) as a critical deleted gene controlling mitochondrial energetics that contributes to the neuronal pathology of 22q11DS. Given the importance of mitochondrial metabolism in the heart, we are investigating the role of SLC25A1 in cardiac function and the impact of SLC25A1 loss on cardiac development and CHD.
Mitochondrial citrate transport: A novel, potentially druggable link to hypoplastic right heart syndrome.
Hypoplastic right heart syndrome (HRHS) is a devastating congenital single-ventricle disease characterized by the underdevelopment of the right side of the heart. Because the molecular etiology of HRHS is not well understood, defining the molecular mechanisms controlling cardiac morphogenesis and identifying specific pathways that contribute to proper heart development are critical to understanding this disease. In the project, we investigate the role of the mitochondrial citrate carrier (SLC25A1) in mitochondrial and ventricular chamber maturation in the developing heart and explore targeting metabolism as a possible avenue to control cardiac morphogenesis and ameliorate SLC25A1-associated congenital heart defects.
Positions Available
Join our team! We are actively recruiting enthusiastic and motivated postdoctoral fellows, Ph.D. students, and laboratory technicians.
If you are interested in joining our lab, please email Jennifer Kwong at jennifer.kwong@emory.edu